あなたはここにいる:ホーム > お知らせ > 企業動向 > Fully Support the R&...
著者:medicilon アップロード:2021-05-07 閲読回数:
Since 2020, the new COVID-19 pandemic has swept the world. With the raging pandemic, the development of the COVID-19 vaccine has become the world's eager anticipation. On November 16 2020, Moderna reported that the effective rate of its mid-term COVID-19 vaccine was 94.5%. On December 2 2020, BioNtech and its US partner, Pfizer, disclosed that the British's Medicines & Healthcare products Regulatory Agency (MHRA) has granted emergency use authorization for the COVID-19 mRNA vaccine, BNT162b2, which is the first approved mRNA vaccine in history and provide the 90% protection rate. These two mRNA vaccines have attracted widespread attention in the industry; therefore mRNA drug research has become a hot field recently.
Medicilon has provided comprehensive support and services for the safety and effectiveness evaluation of various new drugs and vaccines, specifically the research of LNP-mRNA drugs and vaccines, accumulating rich experience. Hence we establish the bioanalysis platform for mRNA vaccines.
mRNA vaccine transport mRNA obtained from target gene expression into cells through a specific delivery system. Then, the mRNA will directly express the target protein in the body to stimulate the specific immune response, , so that the body can obtain protection from the immune system.
mRNA has the below characteristics:
Therefore, the mRNA vaccine has its unique advantages.
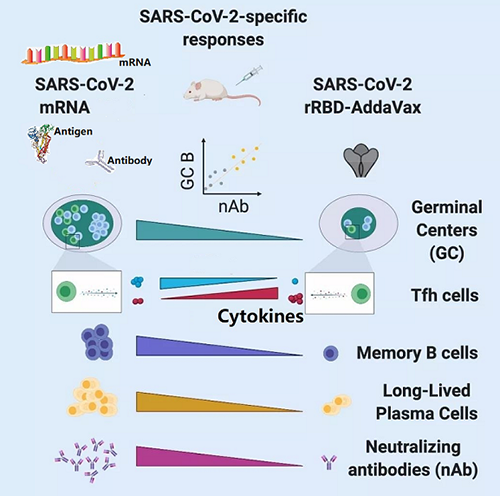
Based on the mechanism and characteristics of mRNA vaccines, Medicilon's Bioanalysis Department has established an evaluation platform covering metabolism and biodistribution, key sequence domain antibodies and antiviral neutralizing antibody titers and the evaluation of effectiveness of cellular immune response.
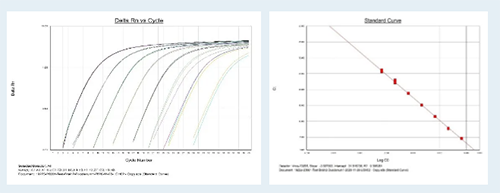
Medicilon equips with the ABI7500 Real-Time PCR System and the Kingfisher Nucleic Acid Extractor. The blood samples stored in Trizol and other types of tissue samples collected from RNAase free tubes can be used for mRNA metabolism and distribution analysis by subsequent RT-qPCR technology.
The mRNA vaccine needs to be expressed to stimulate the body for immune response, so it is particularly important to confirm the expression level and distribution of mRNA at the protein level. Medicilon's Bioanalysis Department has equipped with several M4 Series Microplate Readers and iD5 Series Microplate Readers of Molecular Devices. The MSD series electrochemiluminescence analyzer supports various types of immunoassay methods and could perform detection and monitoring of different sensitivity ranges of protein antigen levels translated by mRNA.

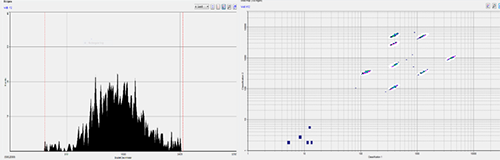
In addition to the production of antibodies by the humoral immune response of B cells, the mRNA vaccine can also stimulate the immune response of cells. The Bioanalysis Department of Medicilon has multiple cytokine analysis platforms such as MSD, luminex and FACS CBA to provide sample-saving and cost-saving cytokine analysis technical support. Medicilon even provides the analysis of ELISPOT single-cell level cytokine abundance change.

The routine evaluation of antibody titer secreted by B cells is mainly based on the indirect ELISA titer detection method. The Medicilon team devoted to the research of nucleic acid and protein vaccine products in the early years, obtained related patents and received a series of trainings on the biological analysis and evaluation of vaccine potency in Pfizer in the United States. Based on practical experience, a series of method evaluation systems and standard-setting rules have been established to investigate the antibody and key domain antibody titers of the vaccine and evaluate their relevance. Base on the MSD platform, Medicilon's Bioanalysis Department could also use the validated kit to perform IgG, IgM, IGA inspection of five different SARS-CoV-2 mRNA expression antigens, SARS-CoV-1 Spike, SARS-CoV-2 N, SARS -CoV-2 S1 NTD, SARS-CoV-2 S1 RBD, and SARS-CoV-2
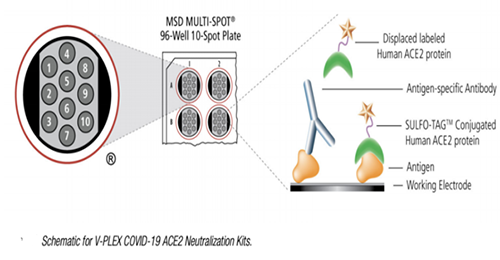
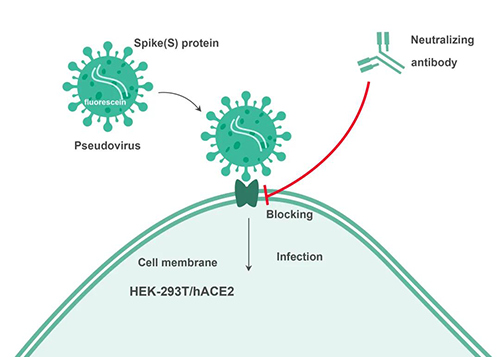
The Medicilon vaccine analysis platform could use the electrochemiluminescence MSD platform to apply competitive ligand binding analysis, such as the analysis of functional antibodies stimulated by the expression of antigens in the new coronavirus mRNA vaccine. There are also facilities such as independent cell rooms for neutralizing antibody analysis based on the interaction between pseudoviruses and cell lines ACE2.
Since the founding of our company in 2004, Medicilon (Stock Code: 688202.SH) has grown into one of the professional drug discovery contract research organizations (CRO) in China. Over the years, Medicilon keeps improving their services in biotechnology and pharmaceutical research. Their services now span across medicinal chemistry, process chemistry, in vitro and in vivo DMPK, preclinical development and bioanalytical support. Medicilon grows together with the clients and delivers the new drug research and development services to more than 700 clients globally. Medicilon is proud to contribute to human health in the globe.